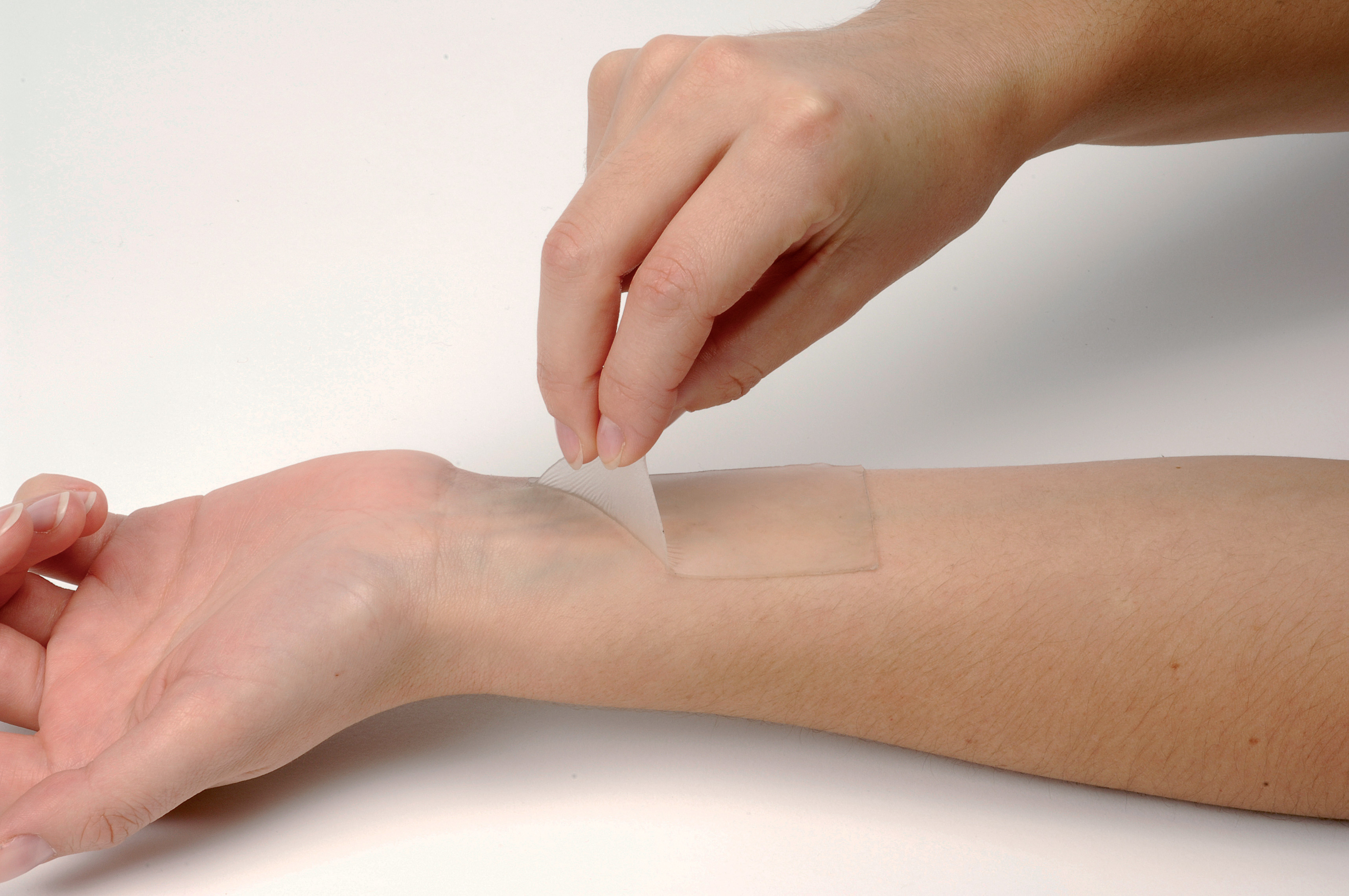
U.S. researchers have developed a fast-acting skin patch that could deliver medications to fight cancer, according to a study released recently.
The study showed that the new skin patch could efficiently deliver medication in one minute to attack cells of melanoma, a deadly form of skin cancer.
Using chicken ovalbumin as a model antigen, the researchers vaccinated mice with their pain-free microneedle patches, and compared the results with intramuscular and subcutaneous injections.
The microneedle treatment produced nine times the antibody level compared to intramuscular injections and 160 times the antibody level compared to subcutaneous injections, the study said.
The researchers also saw efficient immune activation in surgical samples of human skin, it said.
READ ALSO: Ikpeazu reorders contractor to site over poor job
The device is an advance toward developing a vaccine to treat melanoma and has widespread applications for other vaccines, it said.
The study was presented at the American Chemical Society Fall 2019 National Meeting and Exposition by researchers from the Massachusetts Institute of Technology (MIT).
“Our patch has a unique chemical coating and mode of action that allows it to be applied and removed from the skin in just a minute while still delivering a therapeutic dose of drugs,’’ Yanpu He, an MIT PhD student, who helped develop the device, said.
“Our patches elicit a robust antibody response in living mice and show promise in eliciting a strong immune response in human skin,” he said.
“Our patch technology could be used to deliver vaccines to combat different infectious diseases,” Paula Hammond, head of MIT’s Department of Chemical Engineering and leader of the research team, said.
“We are excited by the possibility that the patch is another tool in the oncologists’ arsenal against cancer, specifically melanoma,” she said.
Hammond said the technology will be tested on mice and primates before going to clinic trails. The device is expected to be approved and get on the market in three to five years. (NAN)